Nobel prize awarded for ‘click chemistry’ – an environmentally friendly method of building molecules
The 2022 Nobel prize for Chemistry has been awarded to a trio for developing click chemistry, an environmentally friendly method for rapidly joining molecules to develop cancer treatments, create materials and illuminate the workings of cells

Estimated reading time: 7 minutes
Mark Lorch, University of Hull
Carolyn R. Bertozzi from Stanford University in the US, Morten Meldal from the University of Copenhagen in Denmark, and K. Barry Sharpless from Scripps Research, also in the US, will share the 10 million Swedish kronor (£808,554) award “for the development of click chemistry and bioorthogonal chemistry”.
Chemistry made the modern world, from drugs to synthetic materials, batteries to fuels, flat screens to fertilisers. Often these creations have caused environmental and medical problems, two obvious examples are plastic pollution and health problems associated with “forever chemicals”.
So today chemists are acutely aware of the need to consider the environment and ethical impact of their creations. This has driven scientists to carefully consider how to innovate in a green and sustainable way, while creating new compounds and materials to tackle the world’s challenges.
Building new molecules is hard. It often requires a multitude of sequential individual reactions, each one hampered by side reactions that reduce the purity of the sample. This increases the number and complexity of any further reaction steps, while producing harmful waste that needs careful and expensive disposal.
How it happened
A solution to this problem was conceived by Barry Sharpless at the turn of the millennium. He coined the term “click chemistry”. It’s a concept in which molecules are simply, quickly, reliably and repeatedly joined together in much the same way as a seatbelt clips into its buckle. The idea was the chemical equivalent of the flat-pack wardrobe, while everyone else was building furniture from scratch.
Sharpless also stipulated that click reactions should be carried out in water, instead of harmful solvents commonly used by synthetic chemists to dissolve their reactants. This was a fabulous concept as it would allow quick, reliable and environmentally friendly molecule creation for new products.
But the challenge was making the chemical belts and buckles. The first example of click chemistry was devised by Morten Meldal in 2008 while working on a well studied reaction between two chemicals; azides and alkynes. These are frequently used to join chemicals together, however they normally produce a mucky mess of reactants. But when copper was added to the mix, the reaction produced one, incredibly stable product.
The reaction became extremely popular as it allowed chemists to rapidly change the functionality of a chemical or material. A fibre could have the chemical buckle attached during manufacturing and later extra functionality could be added. The reaction made it easy to click in anti-bacterials, UV protective compounds, or substances that conduct electricity.
In 2004, Carolyn Bertozzi took click chemistry a step further by applying the principle to a biological problem. A common technique for studying the behaviour of molecules in a cell is to attach a fluorescent, glowing label which is clearly visible under a microscope. However, connecting the label to exactly the right part of the cell is tricky.
Bertozzi realised that click chemistry offered a solution. Unfortunately copper, used in Meldal’s original click chemistry method, is toxic to living things so it could not be directly applied to Bertozzi’s problem. Instead she came up with a technique that works without the copper. She attached the azide “buckle” to a sugar molecule. This gets absorbed to the cell, incorporated, and presented on the cell’s surface. A modified alkalyne (the clip) connected to a green fluoresent molecule then gets added to the cell where it clicks to the azide sugar. Then the cell can be easily tracked under a microscope.
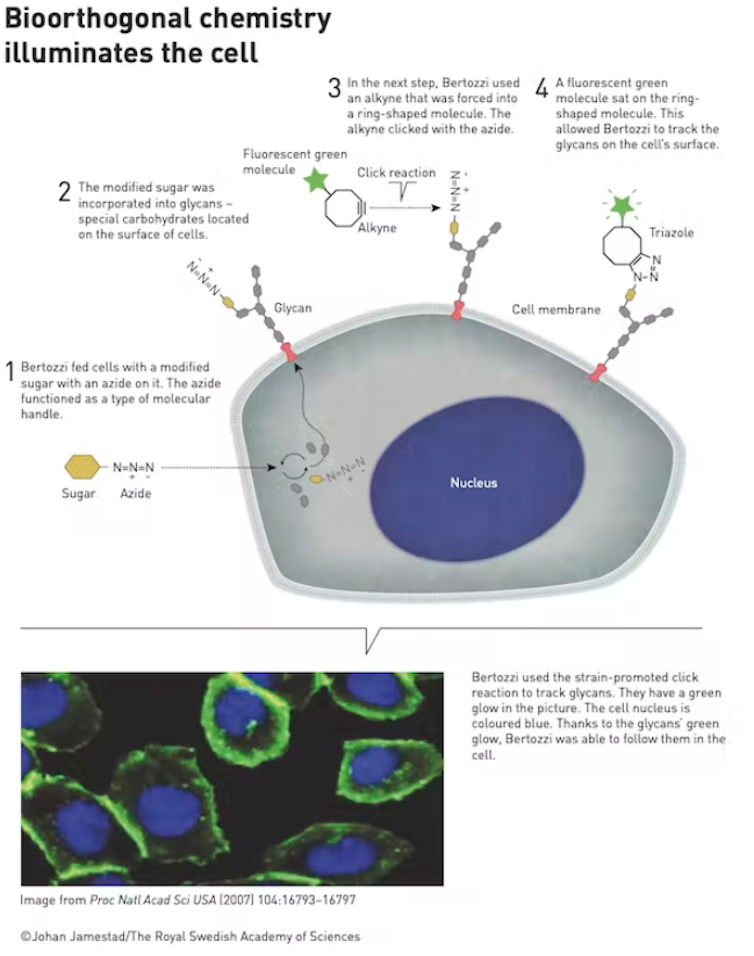
Bertozzi’s technique has led to insights into how tumour cells evade our immune systems and helped develop methods to track cancerous cells. It has also helped to target radiotherapies directly to cancer cells, reducing the harm to nearby healthy cells.
Click chemistry is elegant and efficient. It has allowed chemicals to be joined together almost as smoothly as clicking together two blocks of Lego. Its simplicity has seen its uses spread rapidly through the field of chemistry with applications in pharmaceuticals, DNA sequencing and materials with added functionality (such as magnetic and electrical). There is little doubt the applications of the technique will expand and be applied to the world’s most pressing issues.![]()
Mark Lorch, Professor of Science Communication and Chemistry, University of Hull
This article is republished from The Conversation under a Creative Commons license. Read the original article.
What's Your Reaction?























