Nobel prize in chemistry awarded for ‘quantum dot’ technology that gave us today’s high definition TVs
The 2023 Nobel prize in chemistry has been awarded to a trio for the discovery and development of particles so tiny they were once thought too small to be possible
Estimated reading time: 5 minutes
Laurence Murphy, University of Salford
They are widely used in television screens, LED lights and to guide surgeons removing cancer tumours.
Moungi G. Bawendi from Massachusetts Institute of Technology (MIT) in the US, Louis E. Brus from Columbia University in the US and Alexei I. Ekimov from Nanocrystals Technology Inc. in New York in the US will share the prize sum of 11 million Swedish kronor (£822,910).
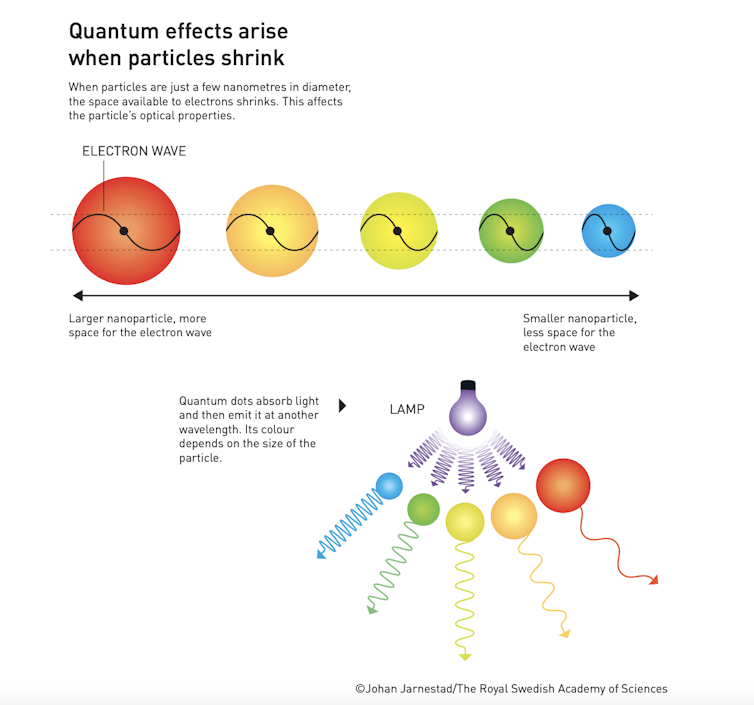
The trio all contributed to the discovery and development of quantum dots, which are nanoparticles (particles between one to 100 nanometres in size) so small that their size actually determines their properties.
Such particles obey the rules of quantum mechanics, governing nature on the smallest of scales, meaning they have optical and electronic properties that are different from those of larger particles.
For example, quantum dots absorb light and emit it at another wavelength – with the resulting colour depending on the particle’s size.
The work started in the early 1980s when Ekimov discovered how to create coloured glass using nanoparticles of copper chloride. A few years later, Brus was the first scientist to prove that nanoparticles in a fluid exhibit quantum effects.
In 1993, Bawendi revolutionised the chemical production of quantum dots, which meant they could be used for practical applications such as in technology and healthcare.
So why are quantum dots so important in the fields of display devices and medical imaging?
As technology for home and commercial use has increased in complexity, so has the resolution and contrast performance of display screens. High definition displays were introduced from 2003 to 2009 where they became the dominant display type available to the public. The successor, ultra high definition, has become today’s standard.
Quantum dots helped increase the range of display colours to more accurately reflect the range of colours the human eye can naturally perceive.
A major problem for technology researchers was how to increase the pallet of colours and sub-colours to do this. Quantum dots give us that flexibility and control.
Quantum dots ultimately offer more accuracy when developing technologies because you can change their properties, such as colour, by changing their size.
Nanotechnology techniques allow us to create molecules of different sizes, to emit different wavelengths of light more accurately and consistently. Quantum dots are bringing us much closer to display screens that reproduce the full range of colours humans can discern.
Quantum dots have been a game changer for medical imaging, too. They have helped create more advanced systems for tumour detection, to study human cells, angiograms (a type of X-ray to examine blood vessels) and even camera-guided surgery and robotic surgery.
Researchers studying the immune system and chemical reactions in the body rely on quantum dots to illustrate their studies more accurately.
We still have not realised the full potential of quantum dots. They have already made their mark on the technology and medical sectors. But they also have the potential to create more accurate imaging for other sectors too, such as astronomy. They might even help create next generation solar cell technology to improve solar cell efficiency for power production.
Not so long ago, we didn’t know quantum dots had different frequencies. Now they are an important part of the technology in our TVs, our lights and the medical science that treats and diagnoses diseases. It’s hard to say how we will be using quantum dots in the future - the limit may be our imagination.![]()
Laurence Murphy, Senior Lecturer & Researcher in Media Technology, University of Salford
This article is republished from The Conversation under a Creative Commons license. Read the original article.
What's Your Reaction?























